
Here's another taster of our Expanded Explanation feature, now available in our Medical Student Finals, MRCP Part 1, MRCP Part 2, MRCP PACES, MSRA, MRCPCH FOP/TAS, MRCPCH AKP, MRCGP (AKT) and MRCS Part A resources. The feature provides a detailed overview of a topic - similar to the one below on Renal Transplantation - alongside related questions in the bank. 
Overview
Key Facts
- In the event of organ failure, transplantation is an option.
- Most commonly performed in the kidney, liver, heart and lung; other organs are increasingly being transplanted.
- Donors may be deceased or alive.
- Issues to be addressed when discussing any organ transplantation are consent, compatibility, immunosuppression and rejection.
- Indication for renal transplant is end stage renal failure to improve quality of life and long-term survival rates when compared to dialysis.
- Patients are at risk of rejection of the kidney, which can occur at any time from the point of transplantation to years afterwards.
- Patients will need lifelong immunosuppression, which in itself can cause complications including infection and malignancy.
- The 1-year graft survival rate is about 94% in live related transplants and 88% in cadaveric transplants.
- The 10-year graft survival rate for first grafts is 70–80%.
- In general, live related recipients fare better than recipients of a cadaveric transplant.
Epidemiology
- There is an increasing frequency of live kidney donation at multiple centres in the United Kingdom (UK).
- Live renal transplantation now accounts for 40% of transplantations.
- In the UK during 2017–18, 3 596 people received a renal transplant.
- In the UK the incidence of end-stage renal failure patients commencing renal replacement therapy is approximately 100/106 people.
- It affects more men than women, and more Afro-Caribbean people than White people.
Aetiology
Causes of chronic renal failure are:
- diabetic nephropathy (34%).
- hypertension (29%).
- glomerulonephritis (14%).
- polycystic kidney disease (14%).
- chronic pyelonephritis (10%).
- obstructive/reflux nephropathy.
Clinical Presentation
Indications for renal transplant
- Renal transplantation is usually carried out for chronic renal failure.
- The advantages of renal transplantation over continued dialysis are:
- improved quality of life (requirement for dialysis reduced or eliminated).
- increase in patient survival rate compared with dialysis (note that selection of fitter patients for surgery may introduce bias).
- cost-effective in the long term.
Patient assessment for renal transplantation
Risk of recurrent disease in the transplanted kidney
- There is a high risk of recurrences in focal segmental glomerular sclerosis, amyloidosis, lgA nephropathy and haemolytic uraemic syndrome.
- Renal transplantation may be inappropriate for live related donors with high recurrence risk of primary disease.
Technical considerations
- Atheromatous iliac vessels in patients with arteriopathy.
- Bladder dysfunction (neurogenic bladder or outflow obstruction).
- Pre-transplantation nephrectomy of a native kidney.
- Adult polycystic kidney disease (APKD): bleeding or infection in cystic spaces.
- Large and bulky kidneys with little space for the transplant.
- Uncontrollable hypertension.
- Renal calculi.
- Persistent anti-glomerular antibodies.
Rejection of transplanted organs
Patients at higher risk of rejection include those who have received previous transplants or multiple blood transfusions, had previous rejection reactions, Afro-Caribbean people and children.
There are several mechanisms of transplant rejection:
- Hyperacute rejection:
- due to presence of recipient antibodies against the donor kidney.
- occurs within minutes of revascularisation.
- kidney swells and becomes discoloured.
- there is clumping of red blood cells (RBCs) and platelets, fibrin is deposited and interstitial haemorrhage occurs.
- rarely seen because of antibody cross-reactivity testing.
- transplant nephrectomy is required.
- Acute rejection:
- defined as an acute deterioration in allograft function that is associated with specific pathological changes in the graft. The two principal forms of rejection are acute cell-mediated rejection and acute antibody-mediated rejection
- acute rejection must be differentiated from other causes of graft dysfunction and/or similar biopsy findings, e.g. post-transplant lymphoproliferative disorder (PTLD) or polyomavirus BK virus, because these may necessitate reduction rather than increase in immunosuppression.
- cell-mediated rejection.
- common in first two weeks but can occur up to six months after transplantation.
- mononuclear cell infiltration occurs in the interstitium and subsequently in vessel walls.
- treatment with high-dose steroids (often reversible).
- difficult to distinguish clinically from acute tubular necrosis (ATN) or drug nephrotoxicity.
- Antibody-mediated rejection (AMR).
- Diagnosis of AMR is provided by the presence of at least three of the following four criteria:
- graft dysfunction.
- histological evidence of tissue injury.
- positive staining for C4d.
- presence of donor-specific antibody.
- Chronic renal transplant rejection:
- is the result of a gradual decrease in the kidney function that starts to become evident three months after the transplantation surgery.
- hypertension and proteinuria are the most important features of declining renal function. Transplant vasculopathy is the single most important feature of chronic renal transplant rejection.
Diagnosis/Investigation
- Donor work-up includes:
- recipient cross-match, screening for transmissible disease.
- magnetic reasonance angiography and isotope renography (the donor keeps the kidney with a greater functional percentage).
- Donor nephrectomy is open via loin incision or laparoscopic (total or hand-assisted).
- Donors usually have normal renal function.
- Additional investigations:
- ABO and HLA typing
- virology (hepatitis B, hepatitis C; CMV which may require treatment pre-transplantation).
- urinalysis and culture.
- consider effects of common co-morbidities (hypertension, diabetes).
- cardiovascular assessment:
- high mortality rate from cardiovascular disease in renal transplant recipients.
- ECG, stress test, echocardiography.
- exclude peripheral vascular disease.
- psychological issues and compliance with lifelong immunosuppressive medication.
Management
Non-pharmacological
- Psychological evaluation and support.
Pharmacological
- At the time of surgery:
- give methylprednisolone 1g.
- anti-CD25 monoclonal antibody (eg basiliximab) selectively targets activated T cells. It is given as an intraoperative injection, which is repeated after four days. The effects last for several weeks and may reduce the levels of ciclosporin or tacrolimus required.
- more aggressive induction agents include alemtuzumab (Campath), anti-thymoglobulin and rituximab, and are the subject of ongoing studies to try to reduce the use of steroids and/or calcineurin inhibitors.
Mechanism of action and side effects of immunosuppressive drugs
Calcineurin inhibitors:
- >ciclosporin: Inhibits production of IL-2 and tumour necrosis factor α (TNF-a) by binding to cyclophilin protein and inhibiting calcineurin.
- side effects:
- nephrotoxicity, hyperkalaemia, hypomagnesaemia, gingival hyperplasia, hyperlipidaemia, glucose intolerance, hypertension.
- side effects:
- tacrolimus: Inhibits production of IL-2 by helper T cells, by binding calcineurin to tacrolimus- binding protein.
- side effects:
- nephrotoxicity, neurotoxicity, glucose intolerance, prolonged Q–T (rare).
- side effects:
- Antiproliferative agents:
- Mycophenolate mofetil: A prodrug. The active compound is mycophenolic acid, which inhibits the enzyme inosine monophosphate dehydrogenase (required for guanosine synthesis); impairs B- and T-cell proliferation selectively because of the presence of guanosine salvage pathways in other rapidly dividing cells.
- side effects:
- nausea, diarrhoea, leucopenia, anaemia and thrombocytopenia.
- side effects:
- Mycophenolate mofetil: A prodrug. The active compound is mycophenolic acid, which inhibits the enzyme inosine monophosphate dehydrogenase (required for guanosine synthesis); impairs B- and T-cell proliferation selectively because of the presence of guanosine salvage pathways in other rapidly dividing cells.
- Azathioprine:
- a derivative of 6-mercaptopurine. It functions as an anti-metabolite to inhibit DNA and RNA synthesis.
- side effects:
- leucopenia, thrombocytopenia, gastrointestinal disturbance, cholestasis, alopecia.
- side effects:
- a derivative of 6-mercaptopurine. It functions as an anti-metabolite to inhibit DNA and RNA synthesis.
Steroids:
- reduce IL-1–3, IL-6 and TNF-a production and inhibit T-cell activation, there is impairment of dendritic cell function (important APCs).
- side effects:
- glucose intolerance, bone disease (osteoporosis, avascular necrosis), cataracts, Cushingnoid appearance, infections, poor wound healing.
- side effects:
mTOR inhibitors (sirolimus/everolimus):
- TOR is a regulatory kinase. Inhibition reduces cytokine-dependent cellular proliferation at the G1–S phase of the cell cycle.
- side effects:
- hyperkalaemia, hypomagnesaemia, hyperlipidaemia, leucopenia, anaemia, impaired wound healing, joint pain.
- side effects:
Maintenance therapy:
- Most units use triple therapy of drugs with synergistic effects, consisting of:
- a calcineurin inhibitor.
- an antiproliferative agent.
- a steroid (prednisolone).
- Other immunosuppressants used are mTOR inhibitors such as sirolimus or everolimus.
Complications of immunosuppression
- Cardiovascular complications (multi-factorial):
- new-onset diabetes after transplantation (NODAT).
- underlying disease causing hypertension, immunosuppressants causing hypertension, hyperlipidaemia.
- Malignancy:
- The incidence of non-skin malignancies in renal transplant recipients is 3.5-fold higher than age-matched controls.
- There is a marked increase (65-fold) in squamous cell carcinoma (SCC) of skin.
- Some malignancies are thought to be related to viral infections, e.g. cervical cancer (human papillomavirus), lymphoma (Ebstein Barr Virus) and Kaposi sarcoma (HHV-8).
- There is a marginal increase in malignancy of solid organs (e.g. breast, colon, lung).
- Problems associated with long-term steroid use:
- hyperglycaemia.
- hypertension.
- thin skin.
- obesity and characteristic fat distribution.
- confusion.
- peptic ulcers.
- poor wound healing.
- Infections:
- opportunistic infections occur in the first few months when immunosuppression is at a maximum.
- CMV infection may occur due to reactivation of endogenous disease or transmission from a donor:
- this infection is very common in the general population, and is usually asymptomatic.
- Prophylaxis (valganciclovir) is given to high-risk recipients (donor CMV-positive to CMV-negative recipients).
- Polyomavirus BK infection is more frequently recognised as an infectious agent in immunosuppressed patients. Can present similarly to rejection and specific stains are required to confirm the diagnosis.
Surgical
Renal transplant surgery
Indications:
- end-stage renal failure.
Pre-op:
- ensure all cross-matching has been performed and is optimum.
- give broad-spectrum prophylactic antibiotics.
- start immunosuppression just before surgery in cadaveric organ recipients. In live donor recipients it may be started a week before surgery.
- the bladder is washed out or filled with approximately 150 ml antiseptic solution.
Procedure:
- an oblique Gibson incision is made in the iliac fossa. The rectus is preserved.
- generally the renal vein is anastomosed at the external iliac vein and the renal artery (with patch) to the external iliac artery.
- during the anastomosis the donor kidney is kept cool on ice or wrapped in a swab soaked with cold saline.
- the ureter is usually anastomosed to the bladder using an extravesical technique. It is tunnelled submucosally in order to prevent reflux. A ureteric stent is placed between the kidney and the bladder (it is removed by cystoscopy at six weeks).
Post-op:
- careful fluid balance is required (usually urine output + 30 ml/hr).
- in situations where the patient remains oliguric or anuric a Doppler scan will assess blood flow and ultrasonography will rule out urinary extravasation or hydronephrosis.
- acute tubular necrosis is associated with increased ischaemia time and may lead to delayed graft function. Renal biopsy may be performed to confirm this and exclude rejection.
Complications:
- vascular: renal artery or vein thrombosis, renal artery stenosis.
- urological: urethral stricture or obstruction.
- lymphocele: due to failure of ligation of lymphatics (can be aspirated).
Kidney transplantation: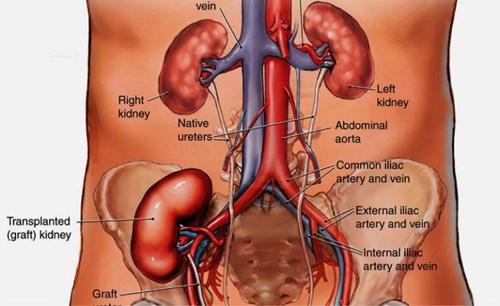
https://www.ucl.ac.uk/immunity-transplantation/clinical-services/diseases-treatments/transplantation/kidney-transplantation
Prognosis
- Prognostic factors include:
- primary diagnosis.
- previous graft failures.
- episodes of rejection.
- kidney total ischaemic time.
- donor factors (e.g. age).
- The 1-year graft survival rate is about 94% in live related transplants and 88% in cadaveric transplants.
- The 10-year graft survival rate for first grafts is 70–80%. In general, live related recipients fare better than cadaveric recipients.
- Non-surgical complications of renal transplantation include:
- complications of immunosuppression.
- primary non-function of unknown cause.
Other
- Contraindications for renal transplantation:
- Active malignancy (cancer-free for at least two years).
- Active infection: exclude dental sepsis and gallstones (risk of cholecystitis).
- Advanced atheromatous disease (relative contraindication).
- Consent issues in organ transplantation:
- The process for organ donation in cadaveric patients is complex and requires a multidisciplinary approach.
- The consent of a live donor involves detailed counselling.
- It is the clinician’s responsibility to ensure that the donor appreciates the short- and long-term implications of organ donation on his or her own health, as well as the risks of failure of the transplant.
Links
- 04 Mar 2022
- Medical Revision